Essential Question
Both the rate (how quickly reactants are converted to products) and the extent (how much reactant is converted to product) are important aspects of chemical reactions. These are important to many fields of science, such as studying how blood clots or how the body breaks down drugs. Factors that influence the rate of reactions include the physical state of the substrate, concentrations, and temperature. In these experiments, students will measure the reaction between copper neocuproine and antioxidants. The results will be compared to trolox, and expressed as the “TEAC value.” TEAC stands for “Trolox Equivalent Antioxidant Capacity.” Trolox is the standard which other antioxidant activity is compared.When mixed, copper chloride and neocuproine form the copper (II) neocuproine complex reagent.The antioxidant they are testing as their sample will reduce copper (II) neocuproine to copper (I) neocuproine. This is a reduction because the oxidation state of copper is reduced in value.$0 $0The absorption of the copper (I) neocuproine will be measured at 450 nm, which they found in the first lab period to be the best wavelength to measure absorbance of neocuproine.Students will then compare the antioxidant activity to that of trolox to obtain the TEAC value. The antioxidant with the highest TEAC value has the highest antioxidant capacity. Students will use this method to compare the antioxidant activity of epicatechin and quercetin to trolox and determine which is the best antioxidant. The larger the TEAC value, the better the antioxidant activity.
Background Information
The original module was created and formatted by: Dr. J. Burgess, in collaboration with The Center for Authentic Science Practice in Education, Purdue University. This resource was modified for Secondary Chemistry by: Dr. V. Frerichs, P. Kresic and S. Small with support from the Dreyfus Foundation Special Grant Program in the Chemical Sciences
Assessment
To calculate the extent of reaction for each antioxidant.To compare the extent of reaction for each antioxidant with that of trolox to determine antioxidant strength.
Student Work
Students will need to calculate the volumes needed to make all dilutions mentioned in Part 2 before coming to lab. Note, they will need to divide dilution and measurement tasks among the group.
Procedure
Turn on the spectrophotometer and allow it to warm up for at least 30 minutes.CUPRAC Procedure Assemble the dilutions of each of the stock solutions of trolox, quercetin and epicatechin. Students should have three dilution solutions of each antioxidant. Each of these will be run in a separate CUPRAC measurement. To provide a blank reference, measure water at 450 nm. For each sample, add the following to a clean test tube:1 ml of copper chloride 1 ml of neocuproine 1mL of buffer 0.75 ml of distilled H2 Then students should add 1mL of the antioxidant they are testing. For the control tube, add 1 ml of ethanol instead of antioxidant. Treat and measure the control sample the same as the experimental samples. Cover the tube with a piece of parafilm and mix by inverting 5 times. Measure the absorbance of the sample at 450 nm using the spectrophotometer. (If they are using cuvettes, they will need to transfer their reaction mixture before measuring.) Students should record the absorbance in the “results” section of their lab write-up. Set the reaction mixture aside, and start a timer. After 20 minutes, take another reading at 450 nm. Record the time of incubation and the absorbance.
Related Resource
volumetric glassware pipettes 50 ml burets for dilution of standard solutions solutions previously made ethanol spectrophotometer cuvets or test tubes Antioxidants Student Manual (PDF)
Instructional/Environment Modifications
1. To calculate the extent of the reaction for trolox, epicatechin, and quercetin, calculate the change (Δ) in the concentration of copper (I) neocuproine using the change in the absorbance you measured between your samples and your control. Note that the reaction stoiciometry shows that for every molecule of copper (II) neocuproine reagent consumed, one molecule of copper (I) neocuproine complex product is produced.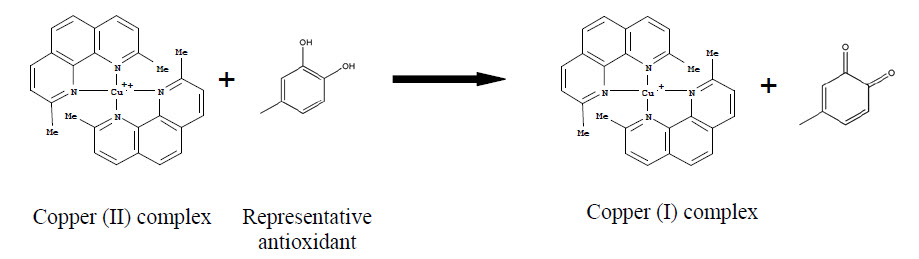 ΔA450 = A450Sample - A450Control measurements at T>20 min where: A450Control = Absorbance at 450 nm of the control sample$0 A450Sample = Absorbance at 450 nm of the reaction [Cu(I)nu] = ΔA450/ e b note: Recall that this is the Beer-Lambert Law molescu(I)nu = ([Cu(I)nu] )(Volume of reaction solution) The following is an example of the data and calculations for this experiment for one antioxidant
ΔA450 = A450Sample - A450Control measurements at T>20 min where: A450Control = Absorbance at 450 nm of the control sample$0 A450Sample = Absorbance at 450 nm of the reaction [Cu(I)nu] = ΔA450/ e b note: Recall that this is the Beer-Lambert Law molescu(I)nu = ([Cu(I)nu] )(Volume of reaction solution) The following is an example of the data and calculations for this experiment for one antioxidant 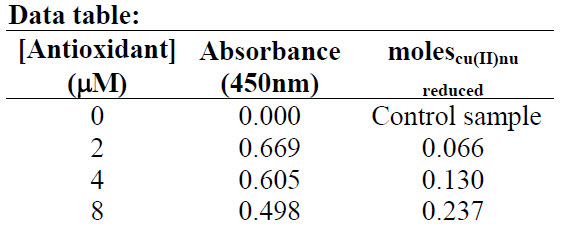 For each antioxidant, make a graph of moles of copper (I) complex product versus antioxidant concentration. Find the slope. If you are using Microsoft Excel© you can use the “add trendline” feature under the chart menu to fit a straight line to the data. Under the options tab, check “set intercept=0,” “display equation on chart,” and “display R-squared value on chart.” The R2 value obtained should be between 0.95 and 1.00 to indicate a good fit of the line to the data. Example of a graph for quercetin:
For each antioxidant, make a graph of moles of copper (I) complex product versus antioxidant concentration. Find the slope. If you are using Microsoft Excel© you can use the “add trendline” feature under the chart menu to fit a straight line to the data. Under the options tab, check “set intercept=0,” “display equation on chart,” and “display R-squared value on chart.” The R2 value obtained should be between 0.95 and 1.00 to indicate a good fit of the line to the data. Example of a graph for quercetin: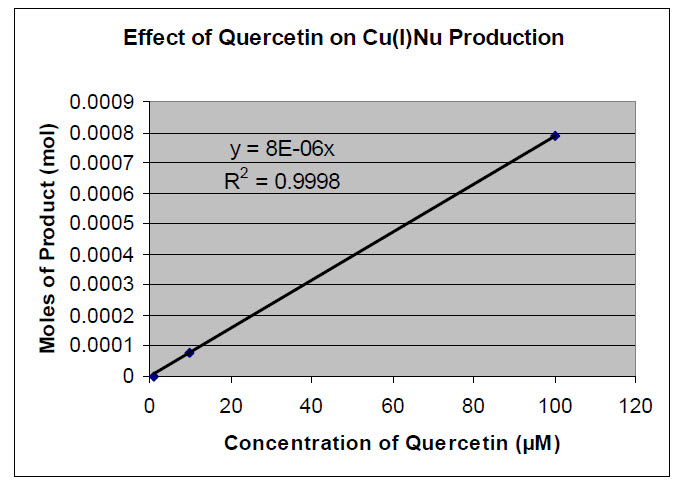 Plot one graph for all three antioxidants. To determine the best antioxidant, you will use the slope of the best-fit line printed on each graph. Divide the slope of the test antioxidants (Quercetin, Epicatechin) by the slope of the Trolox plot to determine the TEAC value.TEAC value = (slope of sample)/(slope of trolox)Because the units are identical in the numerator and the denominator, this value is a unitless ratio when comparing pure compounds of known concentration. 2. Should there be variation in your control absorption values between antioxidants? Was there variation in your experiment? Please explain using complete sentences.3. What TEAC values were obtained for quercetin and epicatechin? Which is a better antioxidant?]4. What was the difference, if any, between the absorbance measured upon mixing and after 20 minutes for each antioxidant? Based on this comparison, which is the stronger antioxidant? Does this agree with your answer to question “3?”
Plot one graph for all three antioxidants. To determine the best antioxidant, you will use the slope of the best-fit line printed on each graph. Divide the slope of the test antioxidants (Quercetin, Epicatechin) by the slope of the Trolox plot to determine the TEAC value.TEAC value = (slope of sample)/(slope of trolox)Because the units are identical in the numerator and the denominator, this value is a unitless ratio when comparing pure compounds of known concentration. 2. Should there be variation in your control absorption values between antioxidants? Was there variation in your experiment? Please explain using complete sentences.3. What TEAC values were obtained for quercetin and epicatechin? Which is a better antioxidant?]4. What was the difference, if any, between the absorbance measured upon mixing and after 20 minutes for each antioxidant? Based on this comparison, which is the stronger antioxidant? Does this agree with your answer to question “3?”
Learning Context/ Introduction
In this lab, students will compare three standard antioxidants using the CUPRAC method. Epicatechin is an antioxidant found in apples, tea, cherries, and grapes. Quercetin is an antioxidant found in fried onions and many other foods. Students will monitor the reaction using spectrophotometry and use their data to estimate the strength of antioxidant activity for quercetin and epicatechin by comparison to trolox.
Assessment
Laboratory 1: Making Solutions and Determining the Best Wavelength of Absorbance for the Copper (I) Neocuproine Complex ProductLaboratory 3: Determination of Ascorbate (Vitamin C) Concentration in Common JuicesLaboratory 4: Independent Research Project
